by Chloé Pelletier
Among these three pictures, I asked forty people in my surroundings to choose their favorite image and the majority chose the chickadee. A lot of people are disgusted by invertebrates, notably by arachnids! But everyone should know how great they are; as Edward O. Wilson said in 1987 they are the “little things that run the world”.

I will try to convince you by sharing my summer project!
My job was to investigate the distribution and the diversity of arthropods across the different woods in the Macpes research forest, in Sainte-Blandine. Samples were harvested during the summers of 2017 and 2018 by the Macpes team. My project was part of Julie-Camille Riva’s master project, to which I collaborated by measuring the biomass of three kinds of arthropods:
- Lepidoptera at both larval and pupal stages
- Araneae
- Hymenoptera, only the Symphyta family at a larval stage (also called sawflies)
These are the main prey of several species of chickadees; here we are focusing on two resident species of the eastern Canada which are the black-capped chickadee and the boreal chickadee (Clements, 2007; Foot et al., 2010). These species eat spiders or caterpillars depending on their availability (Holmes et al., 1979; Robinson et Holmes, 1982), as they are a major food resource that can improve chickadees’ reproductive success (Wilkin et al., 2009). Indeed, in Europe, it has been shown that invertebrate emerge synchronously with chickadee breeding periods, and the number of eggs and viable chicks are correlated with the quantity and quality of food resources, especially caterpillars and spiders (Visser et al., 1998 ; Tremblay et al., 2005; Wilkin et al., 2009).
By identifying the families of invertebrates sampled, we saw which were the most common in Macpes and determine their respective distribution. As we can see in figure 1. A, Tortricidae (36%), known to be damaging insects, and Geometridae (33%), also called looper caterpillars, represented the majority of caterpillars. Araneae constituted mostly of Linyphiidae (26%), which are little spiders very common all over the world (figure 1. B).
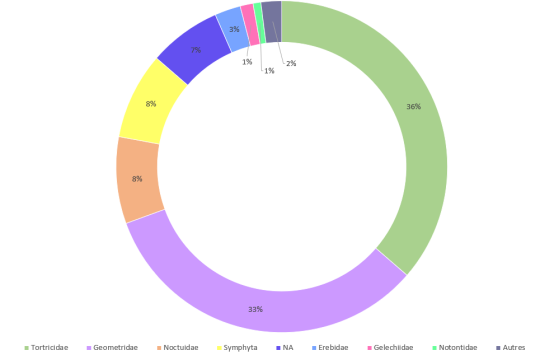
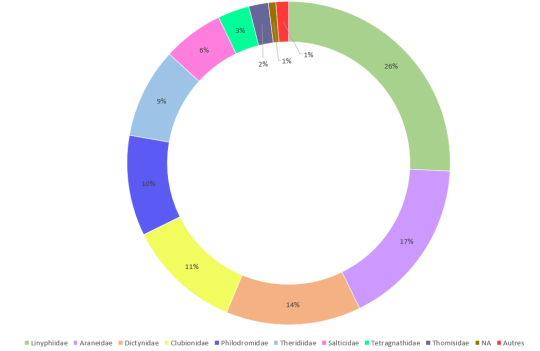
Figure 1. Global taxonomic diversity of the Lepidoptera (A) (and symphyta) and Araneae (B) harvested in 2017 and 2018, Macpes, Sainte-Blandine
It’s important to notice that the spruce budworm (Choristoneura fumiferana) has been present in the Bas-Saint-Laurent since 2012 (MFFP, 2018). This would explain the percent of Tortricidae observed in our samples. Even if spruce budworms are often seen as a threat to our forests, that is not always the case! Let me explain… C. fumiferana mostly attack balsam fir and become detrimental to a forest when found in large numbers and are able to extend their area of occupancy (Morris, 1963). While they defoliate the balsam fir, they allow white spruces to regenerate and that’s what the forest industry is looking for (Baskerville, 1975). However, deforestation can limit the regeneration of white spruce, and caterpillars can thus become allies with the forest industry.
Spruce budworm and other caterpillars are also a very important source of lipids and proteins to insectivorous birds at breeding time (Blondel et al., 1991; Banbura et al., 1999; Vernier et Holmes, 2010). Even though spiders are poor in nutrients, they can also represent an essential part of the chickadees’ diet if they are more abundant than caterpillars near the nest (Holmes et al., 1979; Blondel et al., 1991).
The characterization of arthropod communities is not only essential to better understand insectivorous bird population dynamics, but also to evaluate the quality of their habitats and to position an optimal forest exploitation while conserving biodiversity.

Chloé Pelletier arrived from France in September 2017 to begin a bachelor’s degree in biology at the Université du Québec à Rimouski. She is mostly interested in wildlife and plants conservation and management, but also wishwa to combine social and environmental concerns in my future projects. She worked under the supervision of Christian Nozais during the summer 2019 to do a research training connected with her interests in ecology, conservation and insects.
Bibliography
Baskerville, G. L., 1975. Spruce budworm: super silviculturist. The Forestry Chronicle, 51(4) : 138-140.
Banbura, J., M. M. Lambrechts, J. Blondel, P. Perret et M. Cartan-Son, 1999. Food handling time of Blue Tit chicks: constraints and adaptation to different prey types. Journal of Avian Biology, 263-270.
Blondel, J., A. Dervieux, M. Maistre et P. Perret, 1991. Feeding ecology and life history variation of the blue tit in Mediterranean deciduous and sclerophyllous habitats. Oecologia, 88(1), pp. 9-14.
Clements, J. F., 2007. The Clements checklist of the birds of the world. Cornell Laboratory of Ornithology, & American Birding Association. London: Christopher Helm.
Foote, J. R., D. J. Mennill, L. M. Ratcliffe, et S. M. Smith, 2010. Black-capped chickadee (Poecile atricapillus), version 2.0. The Birds of North America. Cornell Lab of Ornithology, Ithaca, NY.
Holmes, R. T., R. E. Bonney Jr, et S. W. Pacala, 1979. Guild structure of the Hubbard Brook bird community: a multivariate approach. Ecology, 60(3), pp. 512-520.
Morris, R. F., 1963. The dynamics of epidemic spruce budworm populations. The Memoirs of the Entomological Society of Canada, 95(S31) : 1-12.
Ministère des Forêts, de la Faune et des Parcs, 2018. Stratégie de gestion de l’épidémie de la tordeuse des bourgeons de l’épinette. pp. 3-9.
Robinson, S. K., et Holmes, R. T., 1982. Foraging behavior of forest birds: the relationships among search tactics, diet, and habitat structure. Ecology, 63(6), pp. 1918-1931.
Tremblay, I., D. Thomas, J. Blondel, P. Perret et M. M. Lambrechts, 2005. The effect of habitat quality on foraging patterns, provisioning rate and nestling growth in Corsican Blue Tits Parus caeruleus. Ibis, 147(1), pp. 17-24.
Venier, L. A. et Holmes, S. B., 2010. A review of the interaction between forest birds and eastern spruce budworm. Environmental Reviews, 18, pp. 191-207.
Visser, M. E., A. V. Noordwijk, J. M. Tinbergen et C. M. Lessells, 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proceedings of the Royal Society of London. Series B: Biological Sciences, 265(1408), pp. 1867-1870.
Wilkin, T. A., L. E. King et B. C. Sheldon, 2009. Habitat quality, nestling diet, and provisioning behaviour in great tits Parus major. Journal of Avian Biology, 40(2), pp. 135-145.
0 Comments